

A phantom for evaluation of spatial resolution of positron emission tomographs (PET). It is used to characterize the widths of the reconstructed image point spread functions (PSF) of compact radioactive sources. It has been designed in accordance with the NEMA Nu 2-2012.
Locate a local distributor and access exclusive medical offers tailored to your needs.
Kalu Agwu
Address:
2nd Floor Plateau House Plot 79 Ralph Shodeinde Street,
Central Business District, Abuja
Phone:
08181398266
Email:
kalu.agwu@nawegroup.com
For NDT inquiries, please contact:
Address:
54 Drizzel Cres Richmond Hill L4E 1G8
Ontario, Canada
Name:
Jalil Rouzitalab
Phone:
647 804 1348
Email:
jalil@gammafluxndt.ca
Website:
www.gammafluxndt.ca
Address:
B6 Lot 8 & 9 Bethsaida St. Victoriaville Homes
Brgy. Sta. Cruz, Antipolo City
Province Of Rizal 1870
Email:
Phone:
(+63)906-3788179
Website:
Address:
211 Henderson Road,
#13-01 Henderson Industrial Park
Singapore 159552
Phone:
(65) 6273 0487
Fax:
(65) 6278 8687
Email:
Patrick@goldlite.com.sg
sales@goldlite.com.sg
Website:
https://www.goldlite.com.sg/
Non-Destructive Testing
Address:
7952 Nieman Road, Lenexa, KS66214-1560
Phone:
+1 913-685-0675
Email:
Website:
Address:
6910 W Ridge Rd, Fairview, PA 16415
Phone:
+1 877-646-3300
Email:
Website:
Address:
5 Nepperhan Ave Ste 2B, Elmsford, NY10523
Phone:
+1 800-221-0111
Email:
Website:
Address:
P.O. Box 625, Gresham, OR 97030
Phone:
+1 503-907-1925
Email:
Website:
Nuclear Medicine, MRI/CT, Radiography
Address:
PO Box 3468, Youngstown, OH 44513
Phone:
+1 888-9SIRONA
Email:
Website:
Radiography, Fluoroscopy, Mammography, CT, Ultrasound
Address:
3081 Elm Point Industrial Dr, SaintCharles, MO 63301
Phone:
+1 800-242-8428
Email:
Website:
Address:
3120 Deming Way, Middleton, WI53562-1461
Phone:
+1 800-261-4446
Email:
Website:
Radiography, Fluoroscopy, Mammography, MRI, CT, Ultrasound
Address:
P.O. Box 186 Elkhart, IN 46515-0186
Phone:
+1 574-264-4310
Email:
Website:
Antonela Loncar
Phone:
Email:
Ina Günther
Phone:
Email:
Address:
NZ Office: 17b Farnham St, Parnell, Auckland 1052 D: 0800 895 110
website:
email:
Phone:
+35928652628
+359894777684
+359899997953
Email:
184 rue Tabuteau - BP 80345
78533 BUC Cedex (France)
Phone:
+33 (0)1 69 41 10 00
+33 (0)1 69 41 22 41
Website:
Email:
No. 65, Simon Kandelaki St.,
Tbilisi, Republic of Georgia
Phone:
+995 599496110
Email:
Website:
Phone:
+31162729102
+31611186893
+31618063228
+31630743545
Email:
Website:
Phone:
+31162729102
+31611186893
+31618063228
+31630743545
Email:
Website:
Phone:
+351214538756
+351969547972
+351914929518
Email:
Qajaznunu 11/23
0070 Yerevan Armenia
Email:
Website:
Telephone:
+374 99 018070
Kardeljev trg 2,
SI-3320 Velenje,
Slovenia
Phone:
+386 31 35 22 66
Email:
Website:
Address:
Unit 43, Newtown Business & Enterprise Centre, Newtownmountkennedy, Co. Wicklow, Ireland
Contact:
Andrew Martindale
Phone:
+353 1 285 36 59
+353 1 285 53 95 (24 hrs)
Fax:
+353 1 285 43 38
Email:
Website:
Tehran, No. 147 west Nosrat st. Tohid square
Email:
Phone:
+982166590181
+982166590172
+982166424403
Cellphone:
+989381490606
Website:
Our website is multilingual (Persian and English).
Contact name:
Ms. Prisana Virojana
Address:
3/349 C2 Bldg., Popular Rd., Bangpood, Pakkret, Nonthaburi 11120 Thailand
Email:
Phone:
Social Media:
Contact name:
Wara Suwansin, D.Eng. (CTO)
Address:
94/22 Thai Raman Rd., Samwa Tawantok,
Khlong Sam Wa, Bangkok 10510, Thailand
Email:
Phone:
+662 944 3624
Mobile:
+668 6627 4578
Website:
Office no. 102, OASHA Al Marri Building, Al Marrar,
Deira,P.O Box: - 4333
Dubai, UAE
Phone:
+971 52 8515192
Email:
Website:
Store No: 2 & 13, Plot No 1587,
Jurf Industrial Area 2, Ajman, UAE
Phone:
+97155 6680935
Email:
Whatsapp:
+97155 6036746
Address:
1903Ho, 109Dong, 60, Misagangbyeonhangang-ro, Hanam-si, Gyeonggi-do, s.Korea
Phone:
+82(70)4118-3540
Fax:
+82(70)4118-3541
Email:
Website:
Contact Person:
Jin Lee
Address:
José Ureta 632, La Cisterna, Metropolitan Region, Chile
Contact:
Eduardo Espinoza
Email:
Phone:
+562 2511 5192
Fax:
+569 9346 9144
Website:
Address:
Tele Rad Ltda/Sirius Medical
Bogotá D.C.
Phone:
(+57) 316 698 41 69
Email:
Web page:
Skype:
racamacho35
Address:
Adres: 19 Mayıs Mh. Dr. ŞevketBey Sk. No:5
Şişli-Istanbul-Türkiye
Phone:
+90 212 2195657
Fax:
+90 212 2194288
Website:
https://epsilonelektronik.com/
Email:
Address:
Building No55/A,
Street No.53
Ammarat, Khartoum - REPUB. Of Sudan
Phone:
+27833257028
Email:
Sevilla N24-440 y Vizcaya
La Floresta, Quito - Ecuador
Phone:
Email:
Website:
Address:
45 Prime Drive
Seven Hills, NSW 2147
Australia
Phone:
1300 364 336 (Intl. +61 2 8814 5488)
Email:
Contact person:
Jeff Gibson
Address:
Qajaznunu 11/23
0070Yerevan Armenia
Email:
Website:
Phone:
+374 99 018070
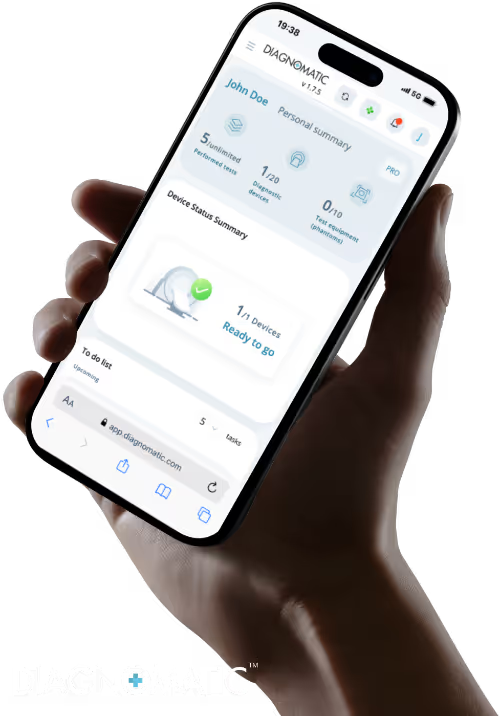
Diagnomatic’s intelligent software automates the testing process through automatic image scoring, detects issues early, guarantees image quality, and ensures equipment compliance, saving time, reducing errors, and strengthening diagnostic confidence.
Supports multi-modality imaging (e.g., CT, MRI, mammography, etc.)
Speeds up accreditation and audit readiness
Facilitates team collaboration and test traceability
Scalable for single sites or enterprise-wide deployments
Instant alerts for missed or failed tests
Automated documentation and audit-ready reports
Flexible deployment: secure cloud access or on-premise installation